What Are The Chemicals In The Frog's Skin Being Studied For?
Abstract
Animals using toxic peptides and proteins for predation or defense typically depend on specialized morphological structures, like fangs, spines, or a stinger, for effective intoxication. Here we show that amphibian poisons instead contain their ain molecular system for toxin delivery to attacking predators. Peel-secreted peptides, more often than not considered part of the amphibian immune arrangement, permeabilize oral epithelial tissue and enable fast access of cosecreted toxins to the predator'due south bloodstream and organs. This absorption-enhancing arrangement exists in at to the lowest degree three distantly related frog lineages and is probable to be a widespread accommodation, determining the outcome of predator–prey encounters in hundreds of species.
Introduction
When a poisonous or venomous creature is attacked by a predator, it commonly has little time to deploy its toxins and induce a response in the aggressor to avoid beingness killed (Fig. 1a). To achieve this, animals may produce toxins with immediate local effects, like pain or distastefulness, or molecules with systemic furnishings that rely on fast infiltration into a predator's body1. In the absence of structures that create a wound to inject toxins, fast delivery is typically only possible for small molecules (<300 Da) that are easily absorbed through oral epithelia, like cyanides, alkaloids, or steroidsi,2. Many frog species however, secrete much larger molecules with systemic targets, like peptides (typically 0.5–ii kDa) and in some cases even small proteins (iv–8 kDa)3,iv,5,six,7. These toxins generally resemble vertebrate hormones or neuropeptides and undergo posttranslational modifications that increase their lifespan in a predator's bloodstream or enhance their affinity to a target receptor8,ix,ten. Upon receptor binding, these toxins substantially simulate an overdose of a predator's hormone or neuropeptide, resulting in a range of adverse effects, including nausea, hypotension, and hyperalgesiathree,7,9,x.
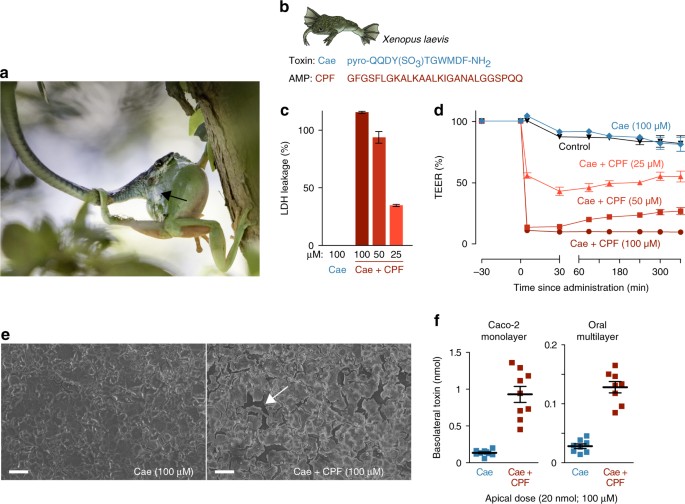
A frog antimicrobial peptide promotes the transepithelial passage of a cosecreted toxin. a Litoria caerulea (an Australian tree frog) attacked by the snake Dendrelaphis punctulatus. Note the defensive skin secretion (black arrow), known to contain toxins (including caerulein) and AMPs (photo taken by Jannie Smit). b Amino-acid sequences of the X. laevis pare toxin caerulein (Cae) and its cosecreted antimicrobial peptide, caerulein precursor fragment (CPF). c Lactate dehydrogenase (LDH) leakage indicates cell damage in a Caco-two epithelial model exposed to a mixture of caerulein and CPF but not to caerulein solitary (n = vii; 100% corresponds to the LDH leakage acquired past complete jail cell lysis every bit induced by Triton-X). d Co-administration of CPF induces a rapid prolonged drib in transepithelial electrical resistance (TEER) of Caco-2 monolayers (n = nine, i-way ANOVA, F(4, 40) = 1345.six, p < 0.0005). due east Caerulein lonely does non impairment Caco-2 monolayers, but co-assistants of CPF results in intercellular ruptures (white pointer), every bit revealed by scanning electron microscopy. Scale confined represent 100 μm. f Co-assistants of CPF at the upmost side leads to higher caerulein levels afterwards 60 min at the basolateral side, indicating enhanced transepithelial transport across Caco-2 monolayers (n = ix, t-test, t(8.ii) = −7.3, p < 0.0005) and an oral multilayer model (n = 8, t-tests, t(9.1) = −9.6, p < 0.0005). All data are mean ± southward.e.m., error bars not shown when covered by data symbols
The large size and physicochemical properties of peptides renders them unsuitable for fast epithelial absorption1,2,11,12,thirteen. The poisons of many frogs thereby seem to defy the constraints of passive toxin uptake, and hint at a mechanism that allows systemic delivery fast enough to evade predation. Besides toxins, many frogs secrete antimicrobial peptides (AMPs), capable of killing a broad range of microorganisms through prison cell lysisxiv,15. Since their showtime characterization in the belatedly 1980s, AMPs have been generally considered a component of the amphibian innate immune system, and investigated primarily in calorie-free of their potency against clinically important pathogens3,14,15,16,17,eighteen. Recently however, an alternative role in antipredator defence force has been suggested, and information technology was predicted that AMPs, through a similar cytolytic action, could permeabilize a predator's epithelial tissue to facilitate toxin delivery7. Proof for this hypothesis would highlight a long-overlooked role of amphibian AMPs besides innate immunity, and drastically alter our perception of how they contribute to an amphibian's survival. Here we report on a series of in vitro and in vivo experiments that provide compelling evidence for an antipredatory role of AMPs. Our results complete the picture of a toxin delivery system every bit sophisticated as that of many venomous animals, exist it molecular rather that morphological in nature.
Results
AMP-enhanced transepithelial passage of a peptide toxin
As the primary model for our study, we used a peptide pair composed of the systemic toxin caerulein, and the AMP caerulein precursor fragment-3 (CPF), both of which are central constituents of the skin secretion of the frog Xenopus laevis 18,nineteen,20 (Fig. 1b). Caerulein is a strong ligand of cholecystokinin receptors expressed in the vertebrate intestine, pancreas, and brain9, and at sufficiently high concentrations, may induce nausea, hypotension, intestinal cramps, airsickness, and diarrheanine,21,22. Fifty-fifty in the intestine, the target receptors are only attainable by ways of epithelial absorption, since they are expressed at the basolateral side (inside the tissue) of the abdominal wall. Because caerulein is a widespread toxin in frogsiii,7,9 with a long half-life in the bloodstream (up to 45 min)8,23, this peptide makes a suitable model to investigate mechanisms of toxin delivery.
The absenteeism of lactate dehydrogenese (LDH) leakage later on applying caerulein (100 μM) to epithelial cell cultures indicate that the toxin is incapable to impairment epithelial cells past itself (Fig. 1c). However, co-administration of caerulein and CPF, resembling the natural status of the frog'due south peel secretion, resulted in dose-dependent LDH leakage within v min indicating rapid and big-calibration damage to cell membranes. Application of CPF solitary produced a similar level of LDH leakage, confirming that this peptide, too lysing bacteria, is capable to perforate epithelial cells (Supplementary Fig. 1a). Accordingly, assistants of CPF and caerulein combined, or CPF lonely, strongly reduced epithelial bulwark integrity equally measured past transepithelial electric resistance (TEER) beyond jail cell monolayers (Fig. 1d; Supplementary Fig. 1b). While application of caerulein alone caused no apparent loss of TEER, exposure to CPF caused an immediate, dose-dependent drib in epithelial barrier integrity. At 100 μM caerulein + 100 μM CPF, TEER dropped to x% ± two.5 of the original value within 5 min. The loss of epithelial integrity is explained by damage to the monolayer that exceeds the mere lysis of private cells (Fig. 1e). The presence of CPF caused abundant intercellular ruptures in the monolayer, indicating that adherent cells were detached, possibly every bit a consequence of CPF-induced cytolysis. This damage allowed the passage of caerulein beyond cell monolayers as confirmed by enzyme-linked immunosorbent analysis (ELISA; Fig. 1f). When caerulein alone (100 μM or 20 nmol) was practical at the apical side of the monolayer (representing the oral fissure), ~ 0.3–1.0% (0.06–0.20 nmol) of the toxin had passed to the basolateral side (representing a predator'due south tissue and claret) subsequently lx min. When CPF was co-administered (at the aforementioned concentration), transepithelial passage of caerulein rose to 2.3–6.8% (0.45–one.36 nmol) of the applied dose, representing a about sevenfold increase on average in the rate of transepithelial passage. Similarly, a nearly fivefold increase in toxin passage was observed when an oral epithelial model composed of 8–eleven stacked cell layers was used, showing the capacity of the AMP to enhance peptide passage through thicker, more complex epithelia (Fig. 1f). Although for both epithelial models, the pct of transferred caerulein remains relatively low, the significant increment caused by the AMP is likely to provide a major reward to a frog under assail, provided that its pare secretion contains a loftier concentration of the toxin (see next section).
AMP-enhanced intoxication of a live predator
Our in vitro experiments provide a strong indication that frog AMPs indeed enhance the transepithelial passage of cosecreted toxins. To test whether this pattern holds in a alive model predator, we quantified radiolabeled caerulein (Supplementary Fig. 2) absorbed past snakes until xxx min later on oral administration in the presence vs. absence of CPF (Fig. 2a). This time window corresponds well with reported times required for snakes and several other predators to subdue and ingest frogs24,25. Despite the long one-half-life of caerulein, radioactivity measurements across such period may increasingly overestimate the amount of intact toxin in a predator'southward body. Nevertheless, the radiolabeled peptide provides a useful tool to quantify and compare the efficiency of epithelial absorption equally the beginning footstep of predator intoxication.
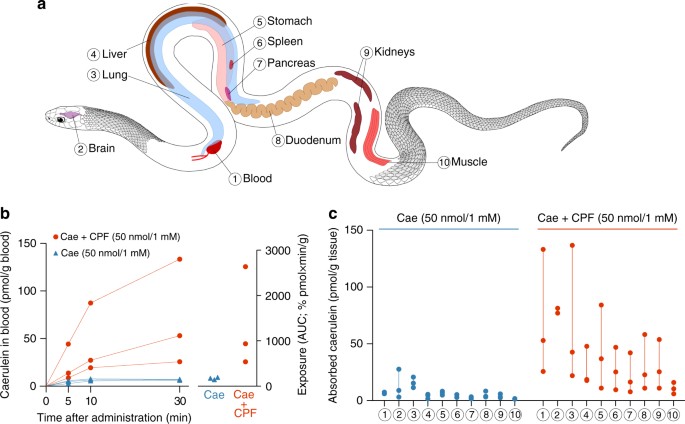
AMP co-assistants accelerates toxin absorption in a live predator. a Investigated organs and tissues in the model predator Thamnophis eques; numbers are cantankerous-referenced in b and c. b Caerulein (Cae) claret concentrations attain higher levels (left graph), which leads to higher systemic exposure (right graph) when orally co-administered with CPF at 1 mM (n = iii, linear mixed model, X ii (ane) = xi.4, p < 0.001). c Caerulein levels in organs are higher in snakes when co-administered with CPF after 30 min (n = iii, linear mixed model, 10 2 (one) = 4.7, p = 0.029). Symbols represent private data values. Linear mixed models are explained in the Methods section
In a first in vivo experiment, we administered both peptides at 1 mM (l nmol in 50 µl buffer solution). This is a biologically realistic concentration for frog peel secretions according to reported estimates for AMPs26,27,28 and ELISA estimates for caerulein (0.12–4.27 mg caerulein per frog, corresponding to 0.088–3.16 μmol, or 1.0–7.5 mM in the pare secretions; Supplementary Fig. three). Monitoring of caerulein levels in the snakes' blood subsequently oral administration revealed a variable only strong effect of the AMP on toxin absorption (Fig. 2b). In the absenteeism of CPF, caerulein claret levels remain low (beneath 8 pmol/k blood) throughout the experiment, but in the presence of CPF, they reach concentrations of xix–87 pmol/g blood after 10 min and 26–133 pmol/g blood after 30 min. Taking into account that a snake's blood takes five–8% of its body weight (BW)29, these blood levels correspond to 0.96–4.36 pmol/g BW after 10 min and 1.28–6.66 pmol/one thousand BW later xxx min. The dose at which caerulein causes adverse effects in snakes is unknown, but the levels observed here largely exceed intravenously injected doses that in previous experiments caused severe sickness in other vertebrates (i μg/kg BW, respective to 0.74 pmol/g BW)9,21,22. Over 30 min, systemic exposure to the toxin (area nether the curve (AUC) derived from repeated blood measurements) ranged upwardly to 13 times college in the presence of CPF (Fig. 2b), and average caerulein levels in diverse organs ranged between 1.3 and 10.four times higher (Fig. 2a, c). The college caerulein levels in all organs suggest that the AMP did not directly the toxin to its specific target organs (intestine, pancreas, and perhaps the encephalon), but instead facilitated dispersal of the toxin throughout the predator'south system by accelerating assimilation. A 2d in vivo experiment, based on the oral administration of 10-fold lower peptide concentrations (100 µM) confirmed that the issue of the AMP is even so apparent at the concentration used in our in vitro experiments. In the presence of CPF, estimated exposure was up to iii times college and boilerplate organ concentrations after xxx min ranged betwixt 1.vii and 3.4 times higher (Supplementary Fig. 4).
A widespread role of frog AMPs in toxin assimilation
AMPs have been characterized in hundreds of frog species, and have been postulated to stand for independent evolutionary origins in distantly related frog lineagesthirty. Besides CPF and related peptides in species of the family Pipidaeeighteen,nineteen,20, AMPs are particularly prominent in the skin secretions of discoglossoid and neobatrachian frogs, in which they, too, co-occur with peptide toxins (Fig. 3a). To investigate whether absorption-enhancing capacity is a shared trait of AMPs from phylogenetically distant frog lineages, nosotros conducted experiments on two boosted peptide pairs (Fig. 3b): The toxin bombesin (Bom) and the AMP bombinin-like peptide one (BLP) from the skin of the oriental burn-bellied toad Bombina orientalis (Discoglossoidea: Bombinatoridae); and the toxin dermorphin and the AMP dermaseptin-S1 (Drs) from the skin of the waxy tree frog Phyllomedusa sauvagii (Neobatrachia: Hylidae). The toxin bombesin is a strong ligand of neuromedin receptors expressed on smooth musculus cells surrounding the gut and claret vessels, which causes hypotension and smooth muscle spasms31,32,33. Dermorphin causes sedation and stupor upon binding its target opioid receptors in the nervous system10,34,35. Similar caerulein, both toxins require access to the bloodstream to induce these effects, and take structural adaptations to extend their half-lives in the bloodstream and tissues34,36.
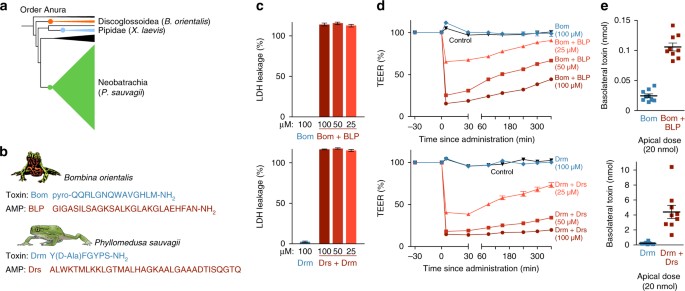
The function of frog AMPs in toxin absorption in other frog species. a Representatives of three major frog lineages cosecrete peptide toxins and AMPs. b Amino-acid sequences of the Bombina ortienalis toxin bombesin (Bom) and AMP bombinin-like peptide 1 (BLP), and of the Phyllomedusa sauvagii toxin dermorphin (Drm) and its AMP dermaseptin-S1 (Drs). c LDH leakage indicates cell damage in Caco-ii monolayers when exposed to the AMP + toxin mixtures, just non to the toxins alone (northward = 6–7; 100% corresponds to the LDH leakage acquired past consummate cell lysis as induced by Triton-X). d BLP and Drs induce a dose-dependent rapid driblet in transepithelial electrical resistance (TEER) of Caco-2 monolayers (northward = 9, ane-style ANOVA, FDRS(four,twoscore) = 1284, FBLP(4,40) = 2054.ane, p < 0.0005). e Co-assistants of BLP and Drs at the apical side of Caco-ii monolayers leads to college toxin levels later on lx min at the basolateral side, showing enhanced transepithelial transport of bombesin (n = ix, t-test, t(eleven.6) = −11.2, p < 0.0005) and dermorphin (due north = 8–9, t-exam, t(8.1) = −four.eight, p < 0.0005). All data are hateful ± s.e.m., error bars not shown when covered by data symbols
Similar to caerulein, neither bombesin nor dermorphin induced any damage to epithelial tissue past themselves (Fig. 3c; Supplementary Fig. 5a). In dissimilarity, co-administration of their corresponding AMPs, or application of the AMPs alone resulted in rapid and severe jail cell leakage and loss of epithelial integrity (Fig. 3d; Supplementary Fig. 5b). BLP and Drs enhanced the transepithelial passage of their cosecreted peptide toxins by ~ four.five and 20 times, respectively (Fig. 3e). These results advise that assimilation-enhancing activity is likely to be a prevalent feature of anuran AMPs, contributing to predator intoxication by a wide range of species.
Discussion
Near inquiry on amphibian skin-secreted peptides has a predominantly pharmacological telescopic, aiming to narrate their physiological effects, non their natural mode of delivery. By investigating the process of intoxication "from a frog'southward perspective", our study has ii major implications.
Commencement, we provide testify for a function of amphibian AMPs outside the immune arrangement. Due to their pore-forming activity and expression in the skin, AMPs are inclined to serve a dual office, in innate immunity equally well equally antipredator defence force. In fact, likewise enhancing the absorption of cosecreted toxins, the cytotoxic activity of amphibian AMPs may cause irritation or pain on oral mucosa, thereby providing a secondary and fast-acting effect on some predators. The wide-spectrum antimicrobial activeness of many amphibian AMPs continues to fuel the perception that they are promising pb compounds for the development of new antibioticsthree,eighteen,37,38. The present evidence however, implies that pharmacological studies seeking to optimize the therapeutic potential of frog AMPs should take their potential damaging effect on epithelial tissue into account.
Second, our results show that toxin systems relying on epithelial assimilation may be more complex than currently perceived, involving multiple factors that together maximize the effectiveness of intoxication. At least in peptide-secreting frogs, epithelial permeabilization is likely to be a decisive step, reducing the time for toxins to reach an agin effect in the predator. The secretion of large amounts of toxin additionally increases the probability that constructive toxin levels are attained in the predator'southward system fifty-fifty if but a small percent of the toxin is absorbed. Similar to the large amounts of caerulein in 10. laevis skin secretion plant hither, quantities ranging upwardly to milligrams accept been reported for toxins in the skin secretion of other frog species, including caerulein in Litoria caerulea (Fig. 1a), and bombesin and dermorphin in Bombina and Phyllomedusa species, respectively10,39,forty. With molecular weights of, respectively, 802.9, 1352.4, and 1619.9 Da, dermorphin, caerulein, and bombesin lie inside the typical size range of anuran peptide toxins. A few toxins however, like anntoxin in Hyla annectans, sauvagine in P. sauvagii, or prokineticins in Bombina are much larger (iv–eight kDa)4,v,half dozen. The question remains whether toxins of this size would also benefit from AMP-enhanced absorption. The degree past which epithelial transfer was enhanced in our in vitro experiments seemed inversely correlated to the toxins' molecular weights, which may indeed indicate that a peptide's size affects an AMP's capacity to mediate its transepithelial uptake.
Absorption enhancement is likely to be effective against predators whose express casualty-treatment skills require considerable time to subdue their casualty, and/or swallow their casualty whole (due east.k., predatory fish, lizards, and large amphibians). In addition, it may human activity in concert with ingestion-delaying behavior such as struggling or inflating. For diverse ophidian species, recorded times between capture and ingestion of amphibians averaged 15–35 min24 and in some cases ranged up to fifty min25. Similarly, amphibians have been documented to survive up to twenty min in the gastrointestinal tract of bullfrogs and fish later beingness swallowed completely25. If in the latter example sickness is induced before the frog dies, it might be regurgitated and escape. Even in predators whose preying technique precludes survival of the frog (e.yard., involving severe trauma before ingestion), enhanced toxin absorption could still adversely affect the predator and be beneficial to the remaining frog population, possibly aided by predator learning25,41,42.
We anticipate that other animals administering their toxins through absorption may employ similar mechanisms of tissue permeabilization. The defense secretions of several whipscorpion species (society Thelyphonida), for case, incorporate caprylic acrid, which, besides increasing the constructive contact expanse of the sprayed secretion, enhances the penetration of cosecreted acetic acid through arthropod cuticle43. Examples involving toxic peptides or proteins with systemic targets may include salamanders44, nemertean worms45, and cone snails releasing insulin-based secretions46. Moreover, the venoms of animals relying on active injection similar biting or stinging (spiders, insects, and cnidarians) frequently include cytolytic agents, which may farther meliorate toxin spreading47,48,49. Every bit such, our findings call for a rethinking of the textbook distinction betwixt poisonous animals (relying on assimilation of their toxins) and venomous ones (creating a wound to guide toxins into the victim)1,2,l,51. Well-known toxin commitment systems range from macroscopic structures (like snake fangs or insect stingers) down to cellular adaptations (nematocysts in cnidarians). The molecular delivery organisation identified hither represents an extension downwards the biological scale, and evidence that toxin commitment can exist mediated at whatever suborganismal level.
Methods
Ideals statement
Experiments involving live animals were conducted in accordance with the European guidelines and the Belgian legislation on animal housing and experimentation, and were approved by the Ethical Committees of Animate being Experimentation of the Vrije Universiteit Brussel (Permit nos. EC15-272-7 and EC16-334-ane) and past the Ethical Commission of the Faculty of Veterinary Sciences of Ghent University (Let no. EC2015/26).
Choice and synthesis of toxins and AMPs
3 toxin–AMP pairs were selected as models for this written report, each representing cosecreted peptides from different clade of frogs: (1) the toxin caerulein (Cae) and the AMP caerulein forerunner factor-iii (CPF) from the skin of the African clawed frog X. laevis (Pipidae); (2) the toxin bombesin (Bom) and the AMP BLP from the skin of the oriental fire-bellied toad B. orientalis (Discoglossoidea: Bombinatoridae); and (3) the toxin dermorphin (Drm) and the AMP dermaseptin-S1 (Drs) from the skin of the waxy tree frog P. sauvagii (Neobatrachia: Hylidae). Sequences and molecular weights of these peptides are provided in the appropriate figures (Figs. 1 and 3). The toxins were selected based on their well-documented systemic effects in vertebratesix,x,31 and on the commercial availability of ELISA kits to allow their in vitro quantification (come across below). The selected AMPs show wide-spectrum antimicrobial action as confirmed by previous studieseighteen,52,53 and are considered part of the frogs' innate allowed organization3,14,15,sixteen,17,eighteen. All peptides were synthesized via standard Fmoc-based solid-stage peptide synthesis past the authors and by CASLO Laboratory ApS (Lyngby, Denmark). For radiolabeling, a caerulein analog, modified with a diethylenetriaminepentaacetic acrid (CHX-A″-DTPA) at the N-terminal side of the peptide (i.e., CHX-A″-DTPA-βAla-Pro-Gln-Asp-Tyr-Thr-Gly-Trp-Met-Asp-Phe-NH2), as a chelator for the isotope indium-111 (Supplementary Fig. 2), was synthesized. Resin-jump peptide (Fmoc-βAla-Pro-Gln(Trt)-Asp(OtBu)-Tyr(OtBu)-Thr(OtBu)-Gly-Trp(Boc)-Met-Asp(OtBu)-Phe-Rink Amide resin) was synthesized manually and subsequently Fmoc deprotection of the N-last β-Ala residue, a solution of CHX-A″-DTPA•3HCl (1.4 eq.) and DIPEA (nine eq) in dichloromethane (DCM)/dimethylformamide (DMF) (2:1, v/v) was added to the resin-jump peptide and the reaction mixture was stirred at room temperature for sixteen h. The resin was washed subsequently with DMF, 2-propanol, and DCM. The complete conversion was verified by Kaiser exam, as well equally by loftier-performance liquid chromatography (HPLC) analysis. The cleavage of the peptides from the resin and the removal of acid-labile protecting groups were achieved using a mixture of TFA/TES/H2O (95:ii.5:2.5, v/v) for 3 h. After evaporation of the cleavage mixture and precipitation in cold Et2O, the crude peptides were purified by semi-preparative reverse phase-HPLC using a mixture of CH3CN/Water, containing one% trifluoroacetic acid (TFA), as mobile stage. The peptides were delivered every bit HPLC-purified ( > 95%) TFA salts. The caerulein-modified peptide containing (CHX-A″-DTPA) was isolated with 7% yield. Nigh peptide salts were dissolved in Milli-Q water to obtain 10 mM peptide stock solutions for storage at four °C.
Prison cell culturing
2 models of gastrointestinal epithelia were selected to represent the mucosal barrier that toxins must cantankerous in lodge to enter the basolateral tissue and blood of a predator. The Caco-2 cell line (Human being malignant intestinal cells, authenticated using light microscopy) was chosen because of its wide application in in vitro epithelial drug transport studies. EpiOral 3D tissues (ordered from Mattek TM, U.s.) were selected to test toxin transport across a more than complex epithelial model equanimous of 8–eleven jail cell layers. Caco-2 cells were cultured in cell medium (87% Dulbecco's modified Eagle's medium nutrient mix, 10% fetal calf serum, 1% not-essential amino acid, 1% kanamycin, 1% penicillin–streptomycin, and 0.1% amphotericin B) in a humidified incubator (ninety% humidity, 5% CO2) at a constant temperature of 37 °C. To prepare an experiment, cultured cells were seeded on the appropriate inserts/wells/coverslips 21 days in advance, at a concentration of ii×x5 cells/ml and further cultured to develop confluent and fully differentiated monolayers (composed cells with polarized apical and basolateral membranes and cell-cell adhesion complexes). The progress of confluence and differentiation was checked by light microscopy and measuring electrical resistance across the cell layer, respectively.
LDH leakage assays
Jail cell-damaging activity of the peptides was tested using commercial LDH Ii assay kits (Abcam, U.s.a.) and Caco-ii prison cell cultures prepared in 96-well plates (run across above), with each well containing 200 µl of medium. For each toxin–AMP pair (see in a higher place), cell cultures were exposed for 5 min to three different AMP + toxin concentrations (100, 50, and 25 µM) and a unmarried toxin concentration (100 µM). Additionally, nosotros tested the effect of merely administering the AMPs at the same 3 concentrations. 9 wells containing cells and medium merely no peptide served as negative controls (representing background LDH release in the absenteeism of cell impairment, or "0% LDH leakage"). Half dozen wells containing 1% triton-X lysis solution were used as positive controls (representing the LDH release caused by 100% cell lysis, or "100% LDH leakage"). Subsequently completing the colorimetric reaction according to the kit's manual, optical density (OD) was measured at 450 nm using a multiskan FC microplate photometer/ELISA reader (Thermo Scientific). The cell-damaging effect of a peptide (in % LDH leakage) was calculated as: 100 × (ODX − ODNorth)/(ODP − ODN), where ODX is the corrected OD value of sample ten, ODN is the average corrected OD obtained for the nine negative control wells, and ODP is the boilerplate corrected OD obtained for the half-dozen positive control wells. Note that the resulting % LDH leakage can exist college than 100%, eastward.yard., if a peptide does non lyse all cells (unlike the total cell lysis in our positive command), leaving the surviving and damaged cells to continue to produce and release additional LDH. Consequently, our LDH leakage assays should be regarded every bit measures of prison cell membrane damage, non cell death.
Monitoring of epithelial permeability
The consequence of peptide assistants on epithelial permeability was investigated by monitoring TEER on Caco-2 cell monolayer cultures. For each toxin–AMP pair (see above), 5 treatments (northward = nine) were monitored: (one) apical administration of the toxin lonely, at 100 μM; (2) apical administration of toxin + AMP, both at 100 μM; (3) toxin + AMP, both at 50 μM; (four) toxin + AMP, both at 25 μM; and (5) no peptide administration (negative command). Caco-two cells were prepared (see in a higher place) on collagen-coated polystyrene transwell insert filters with a 0.four µm pore size (Corning Life Sciences, The states). Inserts were placed in 24-well plates (provided with the inserts), creating an apical compartment (the insert) and a basolateral compartment (the well). Monitoring of TEER was done using a STX2 "Chopstick" electrode connected to an EVOM voltohmmeter (World Precision Instruments Inc). The original TEER (TEERO; respective 100% epithelial integrity), was measured xxx min before administration of the peptides. Peptides were administrated past replacing the apical medium by fresh medium in which the peptides were dissolved. Every bit frequent exposure to electrical currents can damage epithelial cells (explaining the shallow drop of TEER at time 0 in the negative controls in Figs. 1d and 3d), an incubation fourth dimension of minimum 30 min betwixt successive TEER measurements was respected. Hence, TEER was measured 5 min subsequently peptide assistants and so at thirty-min intervals until 3 h later administration, and at 60-min intervals until 6 h later administration. A final measurement was taken at 24 h after peptide administration to monitor recovery of the monolayers after the peptide treatment (data not shown). Relative TEER (in %) values were calculated equally 100 × TEER X /TEERO, where TEER X is the measured TEER at time X.
Scanning electron microscopy imaging
Caco-2 cells were seeded on glass coverslips that were coated with rat tail collagen 1 (Sigma-Aldrich) the day earlier. The effect of four treatments (100 µM CPF, 100 µM caerulein, 100 µM CPF + caerulein, and a negative control) on differentiated Caco-2 monolayers was visualized. Chemical fixation was performed in 12-well culture plates 5 min later on peptide administration (700 µl of solution in all treatments). For fixation, plate wells were filled with i ml 2,5% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.4; 37 °C) for 20 min, and subsequently washed twice with the same buffer. Postfixation was performed with 1% osmium tetroxide for xxx min, followed past three rinses with double-distilled water. Dehydration was carried out using a graded hexylene glycol serial: 30; 50; lxx; and 90%, taking 15 min for each step. Cells were rinsed three times for 10 min with 100% hexylene glycol, and so two more times for 10 min with 100% ethanol. Disquisitional-signal drying of coverslips was done using a Balzers spousal relationship CPD020. Stale coverslips were mounted on carbon-coated stubs with double-sided conductive tape. Gold coating happened with a Jeol 1200JFC fine coater, afterward which coverslips were examined with a Jeol JSM-840 scanning electron microscope operating at 12 kV.
Quantification of transepithelial toxin passage
Toxin concentrations were quantified by ELISA using commercially available kits that prove 100% cantankerous-reactivity with the toxin in question. Caerulein was quantified using the Cholecystokinin Octapeptide (CCK) (26-33) (non-sulfated) ELISA kit (Phoenix Pharmaceuticals Inc., Burlingame, U.s.a.), bombesin was quantified using the Bombesin ELISA kit (Peninsula Laboratories International Inc.), and dermorphin was quantified using the MaxSignal Dermorphin ELISA kit (Biooscientific.com). For each toxin–AMP pair, AMP-induced increase in the passage of the toxin across Caco-ii monolayers was investigated past comparison basolateral concentrations at 60 min after apical administration of either xx nmol of the toxin lonely (yielding a concentration of 100 µM; n = 9), or afterward apical administration of 20 nmol toxin + 20 nmol AMP (100 μM for both; n = 9). Toxin passage across EpiOral multilayered cell cultures was investigated for the caerulein–CPF pair in a similar setup (n = 8). Each basolateral sample was stepwise diluted in cell medium and transferred to an ELISA kit plate. Six wells containing negative control samples (only jail cell medium) and ii wells containing positive command samples (supplied with the kits) were added. Assays were performed following the standard protocols provided with the kits' manuals. Standard curves for each toxin were obtained in duplicate. OD was measured at a wavelength of 450 nm using a multiskan FC microplate photometer/ELISA reader (Thermo Scientific). OD values were converted into toxin concentrations using the four-parameter curve fitting software (Masterplex, MiraiBio Group, Hitachi Solutions America Ltd and elisa-analysis.com).
Quantification of peptides in skin secretion
Previous studies take estimated that AMP concentrations in the skin secretion of various frog species are in the millimolar range26,27,28. For Ten. laevis, AMP concentration estimates range between 4.v and 19.6 mg/ml, and CPF seems i of the virtually abundant peptides18,xix,20. Using CPF every bit a proxy for all 10. laevis AMPs, and given its molecular weight of 2602 Da, these observations would stand for to a CPF concentration of ~1.eight–7.eight mM. The lower limit of this range is slightly above the concentration used in role of our in vivo experiments (see below). Caerulein concentrations in Ten. laevis pare secretions were estimated using ELISA. Adult female person 10. laevis frogs (n = 8) were ordered from a licensed commercial breeder (Xenopus Express France) and housed in plastic containers of 70 × 45 × 45 cm (L×B×H), filled with 25 cm of anile tap water with a filtration system, and kept in a acclimatized animalarium with a 12/12-h day-and-night cycle. To collect skin secretion, each frog was placed in a plastic ziplock bag and manually massaged to simulate the swallowing motion and esophageal peristalsis of a predator (as expected during an attack). The secretion produced during this stimulation tends to stick to the inner surface of the handbag and is therefore easy to collect. Afterward 5 min, all secretion on the frog and the pocketbook's inner surface was collected in a pre-weighed 1.5 ml microcentrifuge tube using a spatula, briefly centrifuged at 10,000 r.p.grand. and weighed again to obtain a net weight for the peel secretion. Obtained pare secretion samples ranged between 85.seven and 419.8 mg, roughly corresponding to 85–420 μl. The secretion samples were subsequently diluted to obtain 10 ml of a 9 M urea/0.1% TFA solution, and fully dissolved by brief vortexing. A 100 μl sample of each solution was used to measure caerulein amounts and concentrations via the ELISA procedure described in a higher place. Using the ELISA results, we inferred that the individual frogs produced between 0.12 and 4.27 mg caerulein, amounts that are high, merely comparable to those estimated for several peptide toxins in other frogs10,39,40. Combined with the estimated skin secretion volumes, the estimated amounts represent to peel secretion concentrations between i.0 and 7.v mM, and the lowest limit of this range was used every bit a biologically realistic concentration for in vivo experiments (see below).
In vivo quantification of absorbed radiolabeled caerulein
To investigate toxin absorption in a alive model predator, radiolabeled caerulein was quantified in the blood and organs of snakes of the species Thamnophis eques (Colubridae: Natricinae) after oral administration in the presence vs. absence of CPF. Adult female person snakes of this species (north = 18) were purchased from J & Thou Garters (Spijkenisse, Holland), and housed in 70 × twoscore × 20 cm plastic boxes (Fifty×B×H), kept in a acclimatized animalarium with a 12/12-h mean solar day-and-nighttime bicycle at 20–25 °C. A beginning experiment was designed to test a biologically realistic peptide concentration, and involved a comparison of 2 treatments (due north = 3): (1) oral administration of 50 µl buffer solution, containing 50 nmol caerulein + 50 nmol CPF (i.e., both at ane mM); and (ii) oral assistants of 50 µl buffer solution containing l nmol (1 mM) caerulein alone. A sample size of three was chosen considering it allows for sufficient statistical power using linear mixed modeling (meet beneath) at a minimum toll of animal lives. A 2nd experiment was designed to test a peptide concentration corresponding to our in vitro tests, which involved the following two treatments (n = half-dozen): (i) oral administration of 50 µl buffer solution containing 5 nmol caerulein + five nmol CPF (i.e., both at 100 μM); and (2) oral assistants of fifty µl buffer solution containing 5 nmol (100 µM) caerulein alone. Here a sample size of six was chosen because we suspected a lower outcome size than in the previous experiment due to the lower peptide concentrations.
For each experiment, snakes were selected for both treatments to obtain identical distributions of BWs. Caerulein-DTPA (65 or 375 nmol) was labeled with 111InCl3 (65–90 MBq) (Mallinckrodt Pharmaceuticals) in 0.2 G NH4OAc (pH 5.0; ammonium acetate) by incubation at 50 °C for 30 min (total volume 413 µl). Radiochemical purity (>95%) was verified past iTLC on silica gel (Drape Corporation) using 0.1 Chiliad sodium citrate buffer (pH 5.0) every bit mobile phase. Snakes were anesthetized with an intracardial injection of propofol (12 µg/thousand BW) and subsequently positioned on their dorsum. Peptide solutions were orally administered with standard syringes by carefully dripping the content (50 µl of a 1 mM or 100 µM buffer solution) on the palate of the snakes, after which the remaining radioactivity of the syringe was measured to determine the radioactivity of bodily administered oral dose. Blood samples (10–50 µl) were taken from the heart with standard insulin syringes at predetermined time points subsequently the start of the experiment (defined as the moment of oral peptide administration). After xxx min, snakes were euthanized with an intracardial injection of T61 (0.5 µg/chiliad BW) and organs and tissues were collected and weighed. Organ and tissue radioactive decay was measured in a gamma counter against a standard of known activity and corrected for the decay in radioactivity. The investigator conducting these measurements was blinded to the oral administration treatments (presence vs. absenteeism of CPF). Before the onset of the experiment, information technology was decided that samples would be excluded from the analysis just if radioactive contamination during the dissection was deemed highly probable. This was the instance for one measurement for the brain (one ophidian treated with i mM caerulein + CPF), where radioactive contagion from the mouth during excision of the brain from the skull could non be ruled out. Caerulein levels in the snakes' blood and organs were expressed in pmol/gram (blood or tissue). Unlike experiments based on intravenous injection, it is impossible to infer accented absorption efficiencies based on the total amount of toxin in a subject field's trunk at any time after oral assistants. Yet, to permit comparison with doses based on whole BWs, as reported in previous studies on caerulein toxicity, the obtained blood values were corrected for the proportion of blood in a snake'south body (5–viii%)29, yielding values in pmol/gram BW. Note that these values may represent an increasingly larger underestimation of the actual amount of toxin absorbed by a snake's body, since an increasingly large fraction of the orally administered dose will have been transferred from the blood to other tissues. Every bit an additional mensurate for systemic toxin exposure, we calculated for each snake the AUC value (expressed in pmol×min/thousand). The AUC value incorporates multiple successive measurements of the amount of toxin in the blood, and is calculated as the expanse under the connecting lines between successive information points as defined by the unremarkably used "trapezoidal dominion" method54,55,56.
Statistics
All data series were tested for normality and equality of variance and afterwards analyzed using SPSS version 23.0 (IBM Corp, USA; most tests) and R version 3.1.ane (https://www.r-project.org; linear mixed models). Differences in TEER amongst peptide treatments were evaluated using one-way assay of variance with a Tukey (equal variances causeless) or Games–Howell (equal variances not assumed) post hoc test. Pairwise differences in transepithelial toxin passage between treatments (ELISA) were evaluated using Student's t-tests. Linear mixed models considering "time" and "treatment" (unlike peptide administrations) as fixed factors and "snake individual" as random factor were used to test for differences in caerulein blood levels over time between treatments. Linear mixed models considering "organ" and "handling" as fixed factors and "snake private" every bit random cistron were used to evaluate their effects on caerulein levels in organs and blood later on xxx min.
Data availability
The data that back up the findings of this report are bachelor from the respective writer upon reasonable request.
References
-
Nelsen, D. R. et al. Poisons, toxungens, and venoms: redefining and classifying toxic biological secretions and the organisms that utilize them. Biol. Rev. 89, 450–465 (2014).
-
Mebs, D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists 1st edn, Ch. 1, 1 (CRC, Boca Raton, 2002).
-
Xu, 10. & Lai, R. The chemical science and biological activities of peptides from amphibian skin secretions. Chem. Rev. 115, 1760–1846 (2015).
-
Mollay, C. et al. Bv8, a small protein from frog skin and its homologue from ophidian venom induce hyperalgesia in rats. Eur. J. Pharmacol. 374, 189–196 (1999).
-
Wei, L. et al. Analgesic and anti-inflammatory effects of the amphibian neurotoxin, anntoxin. Biochimie 93, 995–1000 (2011).
-
Erspamer, V. et al. Sauvagine, a new polypeptide from Phyllomedusa sauvagei skin. Naunyn Schmiedeberg's Curvation. Pharmacol. 312.three, 265–270 (1980).
-
König, E., Bininda-Emonds, O. R. P. & Shaw, C. The diverseness and evolution of anuran skin peptides. Peptides 63, 96–117 (2015).
-
Koulischer, D., Moroder, L. & Deschodt-Lanckman, One thousand. Degradation of cholecystokinin octapeptide, related fragments and analogs by human and rat plasma in vitro. Regul. Pept. 4, 127–139 (1982).
-
Bowie, J. H. & Tyler, Thou. J. in Handbook of Biologically Active Peptides 1st edn, (ed. Kastin, A. J.), Ch. 43, 283–289 (Academic, California, 2006).
-
Negri, L. & Melchiorri, P. in Handbook of Biologically Active Peptides (ed. Kastin, A. J.), Ch. 41, 269–275 (Academic, California, 2006).
-
Moroz, E., Matoori, Due south. & Leroux, J. C. Oral delivery of macromolecular drugs: Where nosotros are afterwards most 100 years of attempts. Adv. Drug. Deliv. Rev. 101, 108–121 (2015).
-
Renukuntla, J., Vadlapudi, A. D., Patel, A., Boddu, Due south. H. Southward. & Mitra, A. K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 447, 75–93 (2013).
-
Caon, T., Jin, L., Simões, C. M. O., Norton, R. S. & Nicolazzo, J. A. Enhancing the buccal mucosal delivery of peptide and protein therapeutics. Pharm. Res. 32, 1–21 (2015).
-
Simmaco, M., Mignogna, Grand. & Barra, D. Antimicrobial peptides from amphibian pare: What do they tell us? Biopolym. Pept. Sci. Sect. 47, 435–450 (1998).
-
Conlon, J. M. Structural diverseness and species distribution of host-defence force peptides in frog peel secretions. Cell. Mol. Life Sci. 68, 2303–2315 (2011).
-
Conlon, J. M. The contribution of skin antimicrobial peptides to the system of innate amnesty in anurans. Cell Tissue Res. 343, 201–212 (2011).
-
Nicolas, P., Vanhoye, D. & Amiche, M. Molecular strategies in biological evolution of antimicrobial peptides. Peptides 24, 1669–1680 (2003).
-
Conlon, J. M. & Mechkarska, G. Host-defense force peptides with therapeutic potential from skin secretions of frogs from the family Pipidae. Pharmaceuticals 7, 58–77 (2014).
-
Richter, K., Egger, R. & Kreil, G. Sequence of preprocaerulein cDNAs cloned from skin of Xenopus laevis. A small family of precursors containing 1, three, or four copies of the final product. J. Biol. Chem. 261, 3676–3680 (1986).
-
Roelants, K. et al. Origin and functional diversification of an amphibian defense peptide arsenal. PLoS Genet. 9, e1003662 (2013).
-
Bertaccini, G., De Caro, G., Endean, R., Erspamer, V. & Impicciatore, M. The actions of caerulein on the polish musculus of the alimentary canal and the gall float. Br. J. Pharmacol. 34, 291–310 (1968).
-
Bertaccini, One thousand., De Caro, G., Endean, R., Erspamer, Five. & Impicciatore, M. The actions of caerulein on the systemic arterial blood pressure of some experimental animals. Br. J. Pharmacol. Chemother. 33, 59–71 (1968).
-
Deschodt-Lanckman, M., Diem Bui, N., Noyer, M. & Christophe, J. Degradation of cholecystokinin-similar peptides by a crude rat brain synaptosomal fraction: a written report by high pressure liquid chromatography. Regul. Pept. 2, 15–30 (1981).
-
Zielinski, W. J. & Barthalmus, G. T. African clawed frog skin compounds: antipredatory effects on African and due north American h2o snakes. Anim. Behav. 38, 1083–1086 (1989).
-
Williams, B. L., Brodie, E. D. Jr. & Brodie, Due east. D. Iii. Coevolution of mortiferous toxins and predator resistance: Cocky-assessment of resistance past garter snakes leads to behavioral rejection of toxic newt prey. Herpetologica 59, 155–163 (2003).
-
Ramsey, J. P., Reinert, Fifty. K., Harper, L. Yard., Woodhams, D. C. & Rollins-Smith, L. A. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 78, 3981–3992 (2010).
-
Gammill, W. M., Scott Fites, J. & Rollins-Smith, L. A. Norepinephrine depletion of antimicrobial peptides from the skin glands of Xenopus laevis. Dev. Comp. Immunol. 37, nineteen–27 (2012).
-
Pask, J. D., Woodhams, D. C. & Rollins-Smith, L. A. The ebb and flow of antimicrobial skin peptides defends northern leopard frogs (Rana pipiens) against chytridiomycosis. Glob. Chang. Biol. 18, 1231–1238 (2012).
-
Bounous, D. I. in Exotic Fauna Medicine for the Veterinarian Technician 3rd edn (eds Ballard, B. & Cheek, R.), Ch. 24, 381 (Wiley, Hoboken, 2016).
-
König, East. & Bininda-Emonds, O. R. P. Show for convergent evolution in the antimicrobial peptide arrangement in anuran amphibians. Peptides 32, 20–25 (2011).
-
Spindel, Eastward. R. in Handbook of Biologically Active Peptides 1st edn (ed. Kastin, A. J.), Ch. 42, 277–281 (Academic, California, 2006).
-
Fox, J. Eastward. T. & McDonald, T. J. Motor effects or gastrin releasing peptide (GRP) and Bombesin in the canine stomach and small intestine. Life Sci. 35, 1667–1673 (1984).
-
Girard, F., Bachelard, H., St-Pierre, S. & Rioux, F. The contractile event of bombesin, gastrin releasing peptide and various fragments in the rat stomach strip. Eur. J. Pharm. 102, 489–497 (1984).
-
Negri, L. et al. Dermorphin and deltorphin glycosylated analogues: synthesis and antinociceptive activeness later on systemic administration. J. Med. Chem. 42, 400–404 (1999).
-
Sander, G. Eastward. & Giles, T. D. Enkephalin analogs and dermorphin in conscious dog: structure-activeness relationships. Peptides three, 1017–1021 (1982).
-
La Bella, R. et al. In vitro and in vivo evaluation of a 99mTc(I)-labeled bombesin analogue for imaging of gastrin releasing peptide receptor-positive tumors. Nucl. Med. Biol. 29, 553–560 (2002).
-
Conlon, J. K., Al-Ghaferi, N., Abraham, B. & Leprince, J. Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents. Methods 42, 349–357 (2007).
-
Rinaldi, A. C. Antimicrobial peptides from amphibian pare: an expanding scenario: commentary. Curr. Opin. Chem. Biol. 6, 799–804 (2002).
-
Roseghini, M., Erspamer, F., Severini, C. & Simmaco, M. Biogenic amines and active peptides in extracts of the skin of thirty-two European amphibian species. Comp. Biochem. Physiol. 94, 455–460 (1989).
-
Anastasi, A., Erspamer, V. & Endean, R. Isolation and amino acrid sequence of caerulein, the agile decapeptide of the skin of Hyla caerulea. Arch. Biochem. Biophys. 125, 57–68 (1968).
-
Nelson, D. M. W., Crossland, 1000. R. & Shine, R. Behavioural responses of native predators to an invasive toxic casualty species. Aus. Ecol. 36, 605–611 (2011).
-
Phillips, B. & Shine, R. When dinner is dangerous: toxic frogs elicit species-specific responses from a generalist snake predator. Am. Nat. 170, 936–942 (2007).
-
Eisner, T., Meinwald, J., Manro, A. & Ghent, R. Defence mechanisms of arthropods—the composition and function of the spray of whipscorpion, Mastigoproctus giganteus. J. Insect Physiol. 6, 272–298 (1961).
-
Jiang, W. et al. Purification and characterization of cholecystokinin from the skin of salamander Tylototriton verrucosus. Zool. Res. 36, 174–177 (2015).
-
Kem, W. in Handbook of Biologically Agile Peptides 1st edn(ed. Kastin, A. J.), Ch. 57, 397–401 (Academic, California, 2006).
-
Safavi-Hemami, H. et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc. Natl Acad. Sci. USA 112, 1–half dozen (2015).
-
Jouiaei, Thousand. et al. Aboriginal venom systems: a review on cnidaria toxins. Toxins seven, 2251–2271 (2015).
-
Vassilevski, A. A. et al. Cyto-insectotoxins, a novel grade of cytolytic and insecticidal peptides from spider venom. Biochem. J. 411, 687–696 (2008).
-
Moreno, Yard. & Giralt, Due east. Three valuable peptides from wasp venoms for therapeutic and biotechnical use: melittin, apamin and mastoparan. Toxins 7, 1126–1150 (2015).
-
Mackessey, Due south. P. et al. in Handbook of Venoms and Toxins of Reptiles 1st edn (ed. Mackessy, S. P.), Ch. 1, 4 (CRC, Boca Ranton, 2009).
-
Fry, B. G. et al. in Venomous Reptiles and Their Toxins 1st edn (ed. Fry, B. One thousand.), Ch. ane (Oxford Univ. Press, New York, 2015).
-
Mangioni, M. L., Fiocco, D., Barra, D., & Simmaco, Thou. in Handbook of Biologically Active Peptides 1st edn(ed. Kastin, A. J.), Ch. 50, 333–337 (Academic, California, 2006).
-
Nicolas, P. & Amiche, G. in Handbook of Biologically Active Peptides 1st edn (ed. Kastin, A. J.) Ch. 45, 295–304 (Academic, California, 2006).
-
Mc.Gready, R. et al. Artesunate/dihydroartemisinin pharmacokinetics in acute falciparum malaria in pregnancy: absorption, bioavailability, disposition and affliction furnishings. Br. J. Clin. Pharm. 73, 467–477 (2012).
-
Ji, H. et al. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal assimilation of curcumin. Drug Deliv. 23, 459–470 (2016).
-
Nielsen, East. J. B. et al. In vivo proof of concept of oral insulin delivery based on a co-assistants strategy with the jail cell-penetrating peptide penetratin. J. Command. Release 189, nineteen–24 (2014).
Acknowledgements
Nosotros thank Jannie Smit for kindly providing the photograph in Fig. 1a, Marjolein Couvreur for help with the scanning electron microscopy, Bram Vanschoenwinkel for help with statistics, and Franky Bossuyt, Keely Smith, Sunita Janssenswillen, and Ines Van Bocxlaer for commenting on before versions of the manuscript. This piece of work is financed by grant no. G0D3214N of FWO-Vlaanderen. Grand.R., Five.C. and South.B. received boosted support from SRP-groeiers grants SRP-xxx of Vrije Universiteit Brussel.
Writer data
Affiliations
Contributions
A.M., F.P., One thousand.R., South.B. and V.C. conceived the project and acquired funding; A.G., C.R., F.P., K.R., Due south.H., T.H. and V.C. designed the research; C.B. and Southward.B. designed and synthesized peptides; C.R. and Due east.Five. conducted the in vitro experiments; C.R., Thousand.1000. and W.B. prepared the scanning electron microscopy; A.Yard., C.P., C.R., F.P., One thousand.R., Due south.H. and T.H. conducted the in vivo experiments; C.R., S.H. and K.R. analyzed the data; all authors contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional data
Publisher's notation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary fabric
Rights and permissions
Open up Access This article is licensed under a Creative Eatables Attribution four.0 International License, which permits employ, sharing, adaptation, distribution and reproduction in any medium or format, equally long as you give appropriate credit to the original author(due south) and the source, provide a link to the Creative Eatables license, and indicate if changes were fabricated. The images or other third party material in this article are included in the article'due south Creative Commons license, unless indicated otherwise in a credit line to the material. If textile is not included in the article's Creative Commons license and your intended use is non permitted by statutory regulation or exceeds the permitted use, you lot will need to obtain permission direct from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Well-nigh this article
Cite this article
Raaymakers, C., Verbrugghe, E., Hernot, S. et al. Antimicrobial peptides in frog poisons constitute a molecular toxin delivery organisation confronting predators. Nat Commun 8, 1495 (2017). https://doi.org/10.1038/s41467-017-01710-one
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1038/s41467-017-01710-one
Further reading
Comments
By submitting a comment you agree to bide by our Terms and Community Guidelines. If you discover something abusive or that does non comply with our terms or guidelines please flag information technology as inappropriate.
What Are The Chemicals In The Frog's Skin Being Studied For?,
Source: https://www.nature.com/articles/s41467-017-01710-1
Posted by: spauldingletly1953.blogspot.com


0 Response to "What Are The Chemicals In The Frog's Skin Being Studied For?"
Post a Comment